Abstract
Introduction: Around 350 new CLL cases are diagnosed annually in Finland, with approximately 1800 individuals living with the disease. However, no comprehensive disease outcome studies from clinical practice have been conducted to-date. In the absence of direct head-to-head trials, robust historical control data have become requisite to complement available clinical trial data and estimate the potential effects of novel therapies in local practice. In addition, patients (pts) in routine clinical care are far more heterogeneous than those included in clinical trials. The Finnish Hematology Registry (FHR) was created to collect real-world practice outcomes in CLL and other hematological malignancies, with Finland's centralized nationwide healthcare system allowing reliable pt identification and complete follow-up. The objective of this registry study is to describe treatment and survival outcomes for CLL pts from routine practice settings.
Methods: A non-interventional, retrospective study design was used to collect routine clinical practice data from the FHR. Eligible pts aged ≥18 years diagnosed with CLL and receiving 1 or more treatment lines during 2005-2015 were identified from the FHR, with groupings of pts into those treated in the early (2005-2007), middle (2008-2010) and late (2011-2015) cohort time periods. As the registry had limited CLL pt data at study start, clinical investigators accessed medical records to retrieve and validate data. We report interim results on 66 pts from the Helsinki University Hospital, Helsinki, Finland, a hospital region accounting for 30% of the total national CLL incidence. The completion and validation of the Helsinki cohort (~130 pts) is ongoing and results from the whole dataset will be presented at ASH.
Results: Median age at diagnosis was 67 years and 23% had Binet stage C. Longitudinal follow-up was available from 1st to 7th or higher treatment lines. Across all treatment lines, fludarabine-cyclophosphamide-rituximab (FCR) (52%), bendamustine-rituximab (BR) (48%), fludarabine-cyclophosphamide (FC) (27%), and chlorambucil (chl) -based therapies (24%) were the most frequently used treatments. FCR (48%) and FC (27%) were frequently used in the 1st line, whereas BR (34%) was the primary choice in the 2nd line. In later lines a wide array of treatment regimens were used. Only 2 pts received the novel agent ibrutinib as part of a named patient program; no other novel agents were used in this cohort during this period.
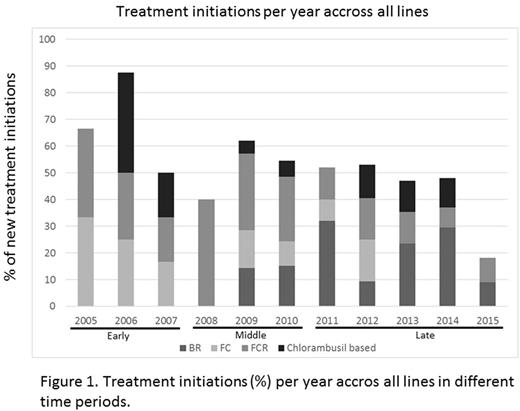
FC and FCR were initiated in equal yet constantly decreasing proportions through 2008, when FCR became most common (0% FC vs. 40% FCR). A year later in 2009, BR was introduced into clinical practice and its use increased steadily from 14% to 30% through 2014 when its use then declined. The peak year of chl monotherapy or chl-based combinations was in 2006 with 38% of pts receiving chl-based treatment. Since then chl use has decreased dramatically, to 0% in 2015.
Median overall survival (OS) from the start of any given 1st line treatment was 81 months (m). Pts receiving FC (p=0,009) and FCR (p<0,001) had significantly longer median OS (79 m and 82 m respectively) compared to bendamustine/BR (62 m) when given in the 1st line. FC and FCR resulted in longer, though not statistically different, time-to-next-treatment (TTNT) compared to bendamustine/BR (77 m vs 44 m). Despite the observed practice changes over the years, there were no detectable outcome differences in 1st and 2nd line median OS between those treated in early, middle and late time periods, possibly due to limited follow-up in the later period (data not shown).
Conclusions: We describe real-world treatment trends and outcomes from a decade of practice in the Helsinki region. Our results show that chemoimmunotherapies have been vigorously implemented in clinical practice. However, interim results do not show detectable improvements for OS and TTNT during this decade of treatment in the Helsinki hospital region. In the Nordics, real-world evidence has become essential when introducing novel targeted therapies into clinical practice settings, with comprehensive disease registries expanding to allow for more detailed follow-up.
Jarvinen: Janssen-Cilag Oy: Employment. Mehtälä: EPID Research: Employment, Other: EPID Research is a contract research organization that performs commissioned pharmacoepidemiological studies and thus its employees have been and currently are working in collaboration with several pharmaceutical companies.. Khanfir: EPID Research: Employment, Other: EPID Research is a contract research organization that performs commissioned pharmacoepidemiological studies and thus its employees have been and currently are working in collaboration with several pharmaceutical companies.. Juuti: EPID Research: Employment, Other: EPID Research is a contract research organization that performs commissioned pharmacoepidemiological studies and thus its employees have been and currently are working in collaboration with several pharmaceutical companies.. Leval: Janssen-Cilag Ab: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal